THE IMPACT OF ALZHEIMER DISEASE ON THE CLINICAL OUTCOME OF COVID-19 A SYSTEMATIC REVIEW

Guilherme Pinho Cardosoa, Bernardo Gabrielle Colaçoa, Bruna Barreto Marque Nunesa, Jucier Gonçalves Júniorb, Modesto Leite Rolim Netoc.
a School of Medicine, Universidade Federal do Ceará – UFC, Fortaleza, Ceara, Brazil
b Department of Internal Medicine, Santa Casa de Misericórdia de Fortaleza, Fortaleza, Ceará, Brazil.
c School of Medicine, Universidade Federal do Cariri – UFCA, Barbalha, Ceara, Brazil
Corresponding Author
Modesto leite Rolim Neto
School of Medicine of Juazeiro do Norte – FMJ/Estácio, Juazeiro do Norte, Ceará, Brazil
Phone: (+55 88 99042979)
Email: modestorolim@yahoo.com.br
ABSTRACT
Introduction: COVID-19 had a great impact on the population, especially on elderly patients. This was especially noticed in patients with chronic comorbid diseases such as diabetes and heart failure, however, other chronic diseases also showed a correlation with the course of the infection, such as Alzheimer's Disease (AD).
Objective: To conduct a literature review on the Impact of Alzheimer's disease on the Clinical Outcome of COVID-19.
Methods: A systematic review was carried out following the PRISMA protocol (Preferred Reporting Items for Systematic Reviews and Meta-Analysis). We have included studies that analyze AD as a Risk factor for worse prognosis or higher mortality of COVID-19.
Results: 8 studies analyzed AD as an important risk factor for mortality from COVID-19. 7 These studies demonstrated a positive correlation between the disease and an increased risk of infection by SARS-CoV-2 and mortality by COVID-19.
Conclusion: There is a possibility that patients with AD develop the severe form of COVID-19. This theory has a biomolecular basis and some observational studies with a good level of evidence support this theory.
Key-words: Alzheimer; COVID-19; Systematic Reviwe.
-
Introduction
Dementia a syndrome that encompasses a group of diseases that have in common the loss of cognitive abilities beyond what is expected for a given age. Globally, it is estimated that 50 million people are living with dementia in its various stages of evolution, ranging from minimal impairment to memory to complete loss of basic skills such as eating (WHO, 2020). Among all the causes of dementia, the most common is Alzheimer's Disease (AD), which has genetic markers and pathophysiological mechanisms well described in the literature and is characterized by a more accentuated impairment of memory in comparison to other cognitive abilities (Thies & Bleiler, 2012).
The World Health Organization (WHO) characterized the disease caused by the SARS-CoV-2 virus as a pandemic on March 11, 2020. Most people with Coronavirus Disease (COVID-19) will develop mild to moderate respiratory illness and recover without requiring special treatment, but older people and those with underlying medical problems like cardiovascular disease, diabetes, and chronic respiratory disease are more likely to experience serious illness (WHO, 2021). Globally, in May 2021 the number of new cases and deaths reported continued to increase, with over 4.1 million new cases and 84 000 new deaths reported, totalizing 167.492.769 confirmed cases of COVID-19, including 3.482.907 deaths (WHO., 2021).
Unfortunately, the issues that COVID-19 provided were not only respiratory but also psychological. In the case of the elderly population, it was possible to correlate the effects of social confinement as contributors and even predisposing to dementia, in which apathy, irritability, suspension, aggression, and depression were the main symptoms identified, and categorized as psychological and behavioral symptoms of dementia (BPSD) (Manini et al., 2021; H. Wang et al., 2021). In this context, there may be a pathophysiological correlation between COVID-19 and the development of AD, the main cause of dementia worldwide. The SARS-CoV-2 virus uses Angiotensin-Converting Enzyme (ACE) as a receptor to enter host cells and the same enzyme is upregulated in the brain of patients with AD. Since the increase in enzyme expression does not depend on the patient's age, a direct relationship between Alzheimer's disease and ACE overexpression can be suggested(Ding et al., 2021). In addition, the increase in the expression of this enzyme may also be related to a state of oxidative stress, which also has an important role in the pathogenesis of AD as shown in Figure 01.
This pathophysiological correlation can lead to important clinical differences between AD patients and some studies point to this disease as a risk factor for increased infectivity and mortality by the new coronavirus, but there is still no evidence in the literature to prove this correlation.
Figure 01: Pathophysiological scheme of the correlation between AD and the SARS-CoV-2.
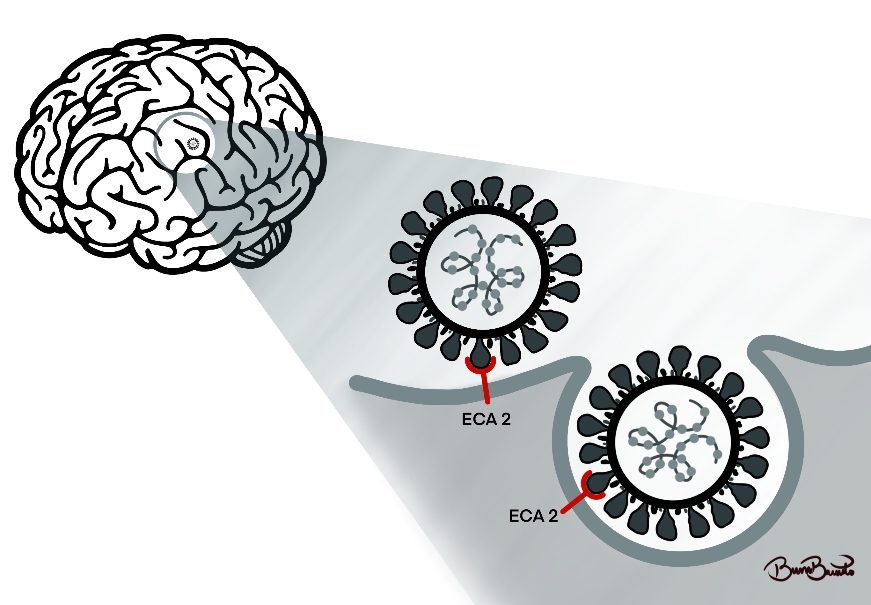
Based on the acronym PICO, which has the following correspondents, P: study population - Patients with AD; I: intervention - demonstrate the effects of COVID-19 in patients with AD; C: control – general population without AD; O: outcome - patients with AD had a worse prognosis in COVID-19 infection.
-
Methods
-
-
Literature review
-
We have performed a systematic review according to the Preferred Reporting Item for Systematic Review and Meta-analysis Protocol (PRISMA) (Moher et al., 2009). We used five electronic databases: Medline, Embase, Scopus, Web of Science, and Cochrane library from the date of inception to May 1, 2021. The electronic searches used variants of the following research terms with a syntax adjusted to each database: #1 “Alzheimer Disease” (Medical Subject Headings – [MeSH term] AND #2 (“COVID-19 (Medical Subject Headings – [MeSH term]”
-
-
Data collection
-
Manuscripts were selected primarily through the analysis of their titles and abstracts. Two researchers collected data individually in order to ensure trustworthiness of the findings, and divergences were solved by a third senior researcher. Each sample article was thoroughly read, and the information was inserted in a spreadsheet (Table 1) that included the author, publishing year, journal, and main findings.
-
-
Eligibility criteria
-
Papers were analyzed based on the following eligibility criteria: at least one combination of the terms described in the search strategy in the title; written in English; addressing the impact of COVID-19 on patients with a previous diagnosis of Alzheimer's Disease; original articles with the full text available through the Journal Portal of the Brazilian Coordination of Personal Improvement of Higher Level (CAPES), which is a virtual library created by the Brazilian Department of Health where content is restricted to authorized users.
Manuscripts repeated in more than one of the databases were counted only once. Some articles were excluded because they did not approach Alzheimer's disease as a part of all types of Dementia. Case reports, Review articles, in vitro or molecular studies, were also excluded. Monographs, dissertations, and thesis were excluded.
-
-
Data presentation and analysis
-
We summarized the results narratively. We described interventions and outcomes based on the information provided in the studies. When studies showed mental health results in numbers without numbers, we extracted them using online software (https://apps.automeris.io/wp d/). We decided not to perform a quantitative analysis of summaries of the associations between the various correlates and health factors, due to a combination of heterogeneity in the measures and lack of control groups, and an embraced lack of descriptions necessary to confirm sufficient homogeneity. We rated the certainty of the evidence using the GRADE approach – (Grading of Recommendations Assessment, Development, and Evaluation
-
-
Ethical issue
-
Considering this is an integrative literature review, Resolution 510/16 of the Brazilian National Health Council (CNS, acronym in Portuguese) ensures the dismissal of submission to an Ethics Committee on Research (Human Beings).
-
Results
Literature search
We searched 634 related studies from all of the mentioned databases, 218 of which were excluded because they were duplicates. The remaining 417 records were filtered based on titles and abstracts, 400 were excluded due to unrelated topics. We reviewed the full text of the remaining 17 studies and identified 8 studies that met the inclusion criteria of the review, including 6 cohort studies, 1 ecological study, and 1 observational case series study.
Description of the selected papers by title, author, and year indexed journal and the main results related to our proposal is shown in Table 01:
Table 01: Summary characteristics of studies included in the systematic review.
AD: Alzheimer's Disease, OR: Odds-Ratio, CI: Control Interval, CMR: Case Mortality Rates.
-
Discussion
As far as we know, this is the first systematic review that analyzed AD as an independent risk factor for a worse prognosis of infection with the new coronaviruses. Studies by Tahira et al (Tahira et al., 2021) and Yu et al (Yu et al., 2021) have shown that patients with A have a higher incidence/morbidity when affected by COVID-19, with AD being an important risk factor in isolation. However, this data seems conflicting when compared to the study by Li et al (Li et al., 2020) in which patients with and without AD who developed pneumonia due to COVID-19 were followed up to assess morbidity and mortality. According to the authors, there was no statistically significant difference between the groups, although only the sample was small, with only 49 individuals.
According to data in the literature, patients with AD have an OR difference of 2.29 compared to their peers with other dementias (OR 2.16). This study included more than 1000 patients who tested positive for COVID-19 from the UK BIOBANK and compared the worst outcomes to a list of 974 different clinical conditions. The most associated with a worse prognosis was AD (Zhou et al., 2021).
The study by Matias-Guiu et al (Matias-Guiu et al., 2020) compared mortality by new coronavirus infection among elderly people with Front Temporal Dementia (FTD) and elderly people with AD. Mortality in the AD group was higher than in the FTD group, but this study is at risk of bias because the elderly with AD in the sample is older than the elderly with FTD, in addition to the low number of patients who tested positive for coronavirus infection (31: 22 with AD and 9 with FTD). In fact, during the first wave, European countries, whose incidence of dementia is high, among them AD, were severely affected. (Hashim et al., 2020).
Other factors that could explain this susceptibility are that patients with dementia have several vulnerabilities to COVID-19 due to social factors (low socioeconomic level, dependence on care, difficulty in adhering to sanitary measures - mask use, social distance, and handwashing), clinical (congenital decline of pathology, advanced age, comorbidities such as chronic non-communicable diseases - systemic arterial hypertension, diabetes mellitus, cardiovascular diseases, chronic obstructive pulmonary disease and neoplasms) (Grippo et al., 2021; Tahira et al., 2021; Wang et al., 2021) and I immunological. Patients with AD can present alterations in the peripheral immune system with a decrease in the number of B and T cells (CD4 + and CD8 +) (Hwang et al., 2020)
The meta-analysis conducted by Liu et al (Liu et al., 2020) suggests a correlation between an increased risk of mortality from COVID-19 in all types of dementia. However, even using such a statistical tool, it was not possible to establish a clear correlation between the diseases due to the heterogeneity of the studies and the low number of publications until the given moment.
Thus, it is understood that in the context of the patient with AD there is, in reality, not a pandemic, in the pure biological context, but a syndemic. This term refers to “a set of closely intertwined and mutual enhancing health problems that significantly affect the overall health status of a population within the context of a perpetuating configuration of noxious social conditions” (Singer, 2000)
During the COVID-19 pandemic period, care for elderly people with comorbidities became even more necessary. Some chronic diseases have a greater correlation with the COVID-19 prognosis, as it appears to be in elderly patients with AD, which may be caused both by the behavior of these patients and by the genetic differences of these patients compared to the general population. It is necessary to reflect on the care of patients with AD, offering greater care to patients with this disease during the pandemic.
Among the limitations found when performing this work, the small number of published studies and without a standardization of analyzed variables. Consequently, the results found were heterogeneous. Therefore, their results should be analyzed with caution. In this sense, future research should be carried out to analyze the effects of COVID-19 in AD patients.
-
Conclusion
According to our systematic review, patients with AD are at an increased risk of becoming infected with SARS-CoV-2, of being hospitalized, and of dying from the infection. The explanation for this correlation may be due to biomolecular changes in AD patients or due to lifestyle habits that have not adapted to the reality brought about by the pandemic.
Finally, there is a possibility that patients with AD are at greater risk of developing severe forms of COVID-19. This theory has a biomolecular basis and some studies build this claim, but more research is needed to prove this correlation. It would be interesting for future studies to compare the different types of dementia with SARS-CoV-2 infection. In addition, these studies should include basic sociodemographic information such as marital status, number of housemates, or perceived emotional support to compare patients according to support social and access to health care in the face of a severe illness.
References
Ding, Q., Shults, N. V., Gychka, S. G., Harris, B. T., & Suzuki, Y. J. (2021). Protein expression of angiotensin-converting enzyme 2 (ACE2) is upregulated in brains with alzheimer’s disease. International Journal of Molecular Sciences, 22(4), 1–14. https://doi.org/10.3390/ijms22041687
Grippo, F., Grande, E., Maraschini, A., Navarra, S., Pappagallo, M., Marchetti, S., Crialesi, R., Frova, L., Orsi, C., Simeoni, S., Carinci, A., Loreto, G., Donfrancesco, C., Lo Noce, C., Palmieri, L., Andrianou, X., Urdiales, A. M., Onder, G., & Minelli, G. (2021). Evolution of Pathology Patterns in Persons Who Died From COVID-19 in Italy: A National Study Based on Death Certificates. Frontiers in Medicine, 8, 645543. https://doi.org/10.3389/fmed.2021.645543
Hashim, M. J., Alsuwaidi, A. R., & Khan, G. (2020). Population risk factors for COVID-19 mortality in 93 countries. Journal of Epidemiology and Global Health, 10(3), 204–208. https://doi.org/10.2991/jegh.k.200721.001
Hwang, J. moon, Kim, J. H., Park, J. S., Chang, M. C., & Park, D. (2020). Neurological diseases as mortality predictive factors for patients with COVID-19: a retrospective cohort study. Neurological Sciences, 41(9), 2317–2324. https://doi.org/10.1007/s10072-020-04541-z
Li, J., Long, X., Huang, H., Tang, J., Zhu, C., Hu, S., Wu, J., Li, J., Lin, Z., & Xiong, N. (2020). Resilience of Alzheimer’s Disease to COVID-19. Journal of Alzheimer’s Disease : JAD, 77(1), 67–73. https://doi.org/10.3233/JAD-200649
Liu, N., Sun, J., Wang, X., Zhao, M., Huang, Q., & Li, H. (2020). The Impact of Dementia on the Clinical Outcome of COVID-19: A Systematic Review and Meta-Analysis. Journal of Alzheimer’s Disease, 78(4), 1775–1782. https://doi.org/10.3233/JAD-201016
Manini, A., Brambilla, M., Maggiore, L., Pomati, S., & Pantoni, L. (2021). The impact of lockdown during SARS-CoV-2 outbreak on behavioral and psychological symptoms of dementia. Neurological Sciences, 42(3), 825–833. https://doi.org/10.1007/s10072-020-05035-8
Matias-Guiu, J. A., Pytel, V., & Matias-Guiu, J. (2020). Death Rate Due to COVID-19 in Alzheimer’s Disease and Frontotemporal Dementia. Journal of Alzheimer’s Disease, 78(2), 537–541. https://doi.org/10.3233/JAD-200940
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., Altman, D., Antes, G., Atkins, D., Barbour, V., Barrowman, N., Berlin, J. A., Clark, J., Clarke, M., Cook, D., D’Amico, R., Deeks, J. J., Devereaux, P. J., Dickersin, K., Egger, M., Ernst, E., … Tugwell, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. https://doi.org/10.1371/journal.pmed.1000097
Singer, M. (2000). A dose of drugs, a touch of violence, a case of AIDS: conceptualizing the SAVA syndemic. Free Inquiry in Creative Sociology, 28(1), 13–24.
Tahira, A. C., Verjovski-Almeida, S., & Ferreira, S. T. (2021). Dementia is an age-independent risk factor for severity and death in COVID-19 inpatients. Alzheimer’s and Dementia. https://doi.org/10.1002/alz.12352
Thies, W., & Bleiler, L. (2012). 2012 Alzheimer’s disease facts and figures. Alzheimer’s and Dementia, 8(2), 131–168. https://doi.org/10.1016/j.jalz.2012.02.001
Wang, H., Qin, R., Zhang, J., & Chen, Y. (2021). Possible immunity, inflammation, and oxidative stress mechanisms of Alzheimer’s disease in COVID-19 patients. Clinical Neurology and Neurosurgery, 201. https://doi.org/10.1016/j.clineuro.2020.106414
Wang, Q. Q., Davis, P. B., Gurney, M. E., & Xu, R. (2021). COVID-19 and dementia: Analyses of risk, disparity, and outcomes from electronic health records in the US. Alzheimer’s and Dementia. https://doi.org/10.1002/alz.12296
WHO. (2021). Weekly epidemiological update on COVID-19 - 25 May 2021. Weekly Epidemiological Update on COVID-19 - 25 May 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---25-may-2021
WHO. (2020). Dementia. https://www.who.int/news-room/fact-sheets/detail/dementia
WHO. (2021). Coronavirus. Coronavirus. https://www.who.int/health-topics/coronavirus#tab=tab_1
Yu, Y., Travaglio, M., Popovic, R., Leal, N. S., & Martins, L. M. (2021). Alzheimer’s and parkinson’s diseases predict different COVID-19 outcomes: A UK biobank study. Geriatrics (Switzerland), 6(1), 1–15. https://doi.org/10.3390/geriatrics6010010
Zhou, J., Liu, C., Sun, Y., Huang, W., & Ye, K. (2021). Cognitive disorders associated with hospitalization of COVID-19: Results from an observational cohort study. Brain, Behavior, and Immunity, 91, 383–392. https://doi.org/10.1016/j.bbi.2020.10.019








